Abstract

Introduction: Despite recent advances in the treatment of follicular lymphoma (FL), patients frequently require multiple lines of therapy, and an unmet need remains for patients who experience relapsed or refractory (R/R) disease. After a median follow-up of 27.0 months (safety-evaluable population), the novel triplet combination of polatuzumab vedotin (Pola) plus obinutuzumab (G) and lenalidomide (Len) was shown to be efficacious in a heavily pre-treated and refractory FL population and had a safety profile consistent with known profiles of the individual drugs (Diefenbach C, et al. Lancet Haematol 2021). Here, we report the final analysis with updated safety and efficacy data for this combination.
Methods: GO29834 (NCT02600897) was an open-label, multicenter study of patients with R/R FL (excluding Grade 3b) who had received ≥1 prior anti-CD20-containing chemoimmunotherapy regimen. The recommended Phase II dose for Pola and Len in combination with G was established in an initial 3+3 dose-escalation phase and expanded into a Phase II study. Patients in the expansion cohort received induction treatment with six 28-day cycles of: intravenous (IV) G 1000mg (Cycle [C]1: Day [D]1, 8, 15; C2-6: D1); IV Pola 1.4mg/kg (D1), and oral Len 20mg (D1-21). Responders and those with stable disease received maintenance treatment for 24 months (G 1000mg on D1 every 2 months and Len 10mg on D1-21 of Months 1-12). The primary endpoint was complete response (CR) at end of induction (EOI), determined by the Independent Review Committee (IRC) based on positron emission tomography-computed tomography scans by modified Lugano 2014 criteria (Cheson BD, et al. J Clin Oncol 2014). Secondary endpoints included progression-free survival (PFS), overall survival (OS), and safety.
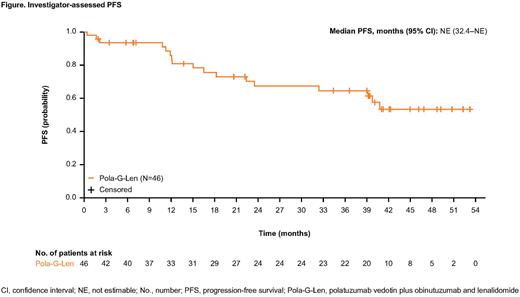
Results: A total of 56 patients were enrolled across Phase Ib and Phase II; median duration of follow-up was 43.5 months in the safety-evaluable population at the time of final analysis (March 03, 2022). Baseline characteristics were: median age, 62 years; male, 59%; Ann Arbor Stage III-IV, 88%; Follicular Lymphoma International Prognostic Index 1 high-risk (≥3), 55%; bulky disease (≥7cm), 16%; median prior lines of treatment, 3 (range: 1-7); ≥2 prior lines of therapy, 77%; refractory to last line of prior regimen, 59%; refractory to last line of anti-CD20 treatment, 55%; and POD24 (defined as disease progression within 24 months of initiation of first anti-lymphoma treatment with chemoimmunotherapy), 27%. All patients (N=56) had at least one adverse event (AE), 36 (64%) had a serious AE, and 48 (86%) had a Grade 3-4 AE. The most common Grade 3-4 AEs were neutropenia (n=31, 55%), thrombocytopenia (n=14, 25%), infections (n=14, 25%), and anemia (n=8, 14%). AEs leading to dose reduction or interruption of any drug occurred in 18 (32%) and 43 (77%) patients, respectively; the majority were modifications of Len. In addition, 19 (34%) patients had an AE that led to discontinuation of any study drug. Three Grade 5 (fatal) AEs were reported (one case of septic shock and two cases of COVID-19). In the primary efficacy population (N=46), the IRC-assessed objective response rate at EOI was 76%, with a CR rate of 61%. The best overall response rate by investigator assessment (INV) was 91%, with a best CR rate of 72%. After a median follow-up of 43.3 months in the efficacy-evaluable population (N=46), median PFS was not reached (Figure). Landmark PFS by INV was 83% (95% confidence interval [CI]: 72-95) at 12 months, 67% (95% CI: 53-82) at 24 months, and 53% (95% CI: 36-70) at 48 months. Median OS was not reached.
Conclusions: In the GO29834 study, we evaluated the safety and efficacy of novel triplet combination Pola-G-Len in patients with R/R FL, a population for whom there is a continued unmet medical need. This final analysis demonstrates a safety profile consistent with the known profiles of the individual drugs. High CR rates at EOI in a heavily pre-treated and refractory population compare favorably with currently available R/R FL therapies. After a median follow-up of 43.3 months in the efficacy-evaluable population, the median PFS and OS were not reached, and more than half of the patients treated with Pola-G-Len had not experienced disease progression after 4 years.
Disclosures
Diefenbach:Merck: Consultancy, Research Funding; MEI Pharma: Research Funding; Kite: Consultancy; Incyte: Research Funding; IMAB: Consultancy; Gilead: Current equity holder in publicly-traded company; Genmab: Consultancy; Genentech/Roche: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Celgene: Consultancy; BMS: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; Seattle Genetics: Research Funding. Kahl:AstraZeneca: Consultancy, Research Funding; ADT Therapeutics: Consultancy; Roche: Consultancy; Genentech: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; MEI: Consultancy; AcertaPharma: Consultancy; Pharmacyclics: Consultancy; Celgene/BMS: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Kite: Consultancy; Janssen: Consultancy; Incyte: Consultancy; Hutchmed: Consultancy, Research Funding; TG Therapeutics: Consultancy; Genmab: Consultancy; Seattle Genetics: Consultancy; Research To Practice: Speakers Bureau. McMillan:Amgen: Honoraria; Roche: Honoraria; Takeda: Honoraria, Other: Travel funding; Prosethetics: Honoraria. Miall:Roche: Honoraria; Takeda: Honoraria. Briones:BMS: Consultancy, Honoraria; Gilead: Consultancy; Novartis: Consultancy, Honoraria; GSK: Consultancy; Takeda: Consultancy, Honoraria; Celgene/BMS: Research Funding; HOSPITAL SANTA CREU I SANT PAU: Current Employment. Cordoba:Celgene: Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Pfizer: Research Funding; Incyte: Consultancy; GenMab: Consultancy; Takeda: Consultancy; Kite: Consultancy, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; BeiGene: Consultancy; Lilly: Consultancy; Gilead: Honoraria; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau. Burke:Abbvie: Consultancy; Adaptive Biotechnologies: Consultancy; AstraZeneca: Consultancy; BeiGene: Consultancy, Speakers Bureau; BMS: Consultancy; Epizyme: Consultancy; Kura: Consultancy; Kymera: Consultancy; Morphosys: Consultancy, Research Funding; Nurix: Consultancy; Roche/Genentech: Consultancy; SeaGen: Consultancy, Speakers Bureau; TG Therapeutics: Consultancy; Verastem: Consultancy; X4 Pharmaceuticals: Consultancy. Hirata:Roche: Current holder of stock options in a privately-held company; Genentech, Inc.: Current Employment. Sharma:Syneos Health: Current Employment; Zydus lifesciences Ltd: Ended employment in the past 24 months. Musick:Roche/Genentech: Current equity holder in private company, Current holder of stock options in a privately-held company; Genentech: Current Employment. Abrisqueta Costa:Sandoz: Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; Bristol-Myers-Squibb: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau.
OffLabel Disclosure:
Polatuzumab vedotin is an antibody-drug conjugate targeting CD79b on malignant B-cells. Polatuzumab vedotin in combination with bendamustine and rituximab (Pola-BR) is approved by the FDA for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma, not otherwise specified, after at least two prior therapies. Obinutuzumab (Gazyva) is a CD20-directed cytolytic antibody indicated: in combination with chlorambucil, for the treatment of pts with previously untreated CLL; in combination with bendamustine followed by obinutuzumab monotherapy, for the treatment of pts with FL who relapsed after, or are refractory to, a rituximab-containing regimen; in combination with chemo followed by obinutuzumab monotherapy in pts achieving at least a PR, for the treatment of adult pts with previously untreated stage II bulky, III or IV FL.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal